

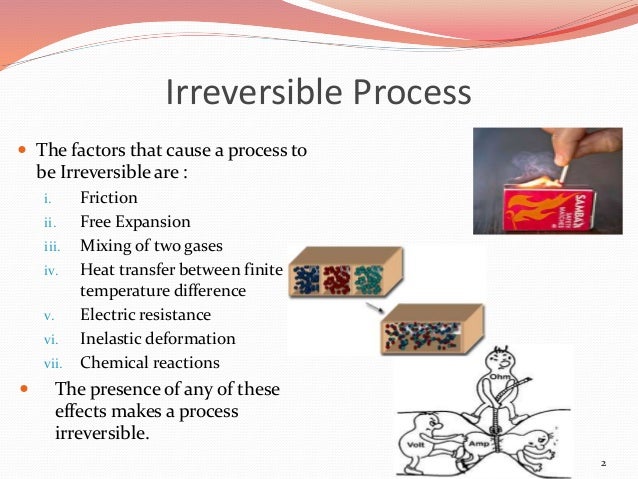
Note that the idea of a reversible process is a formalism required to support the development of various thermodynamic concepts no real processes are truly reversible, rather they are classified as irreversible. The term reversible process refers to a process that takes place at such a slow rate that it is always at equilibrium and its direction can be changed (it can be “reversed”) by an infinitesimally small change is some condition. This new property was expressed as the ratio of the reversible heat ( q rev) and the kelvin temperature ( T). In a later review of Carnot’s findings, Rudolf Clausius introduced a new thermodynamic property that relates the spontaneous heat flow accompanying a process to the temperature at which the process takes place. In 1824, at the age of 28, Nicolas Léonard Sadi Carnot ( ) published the results of an extensive study regarding the efficiency of steam heat engines.
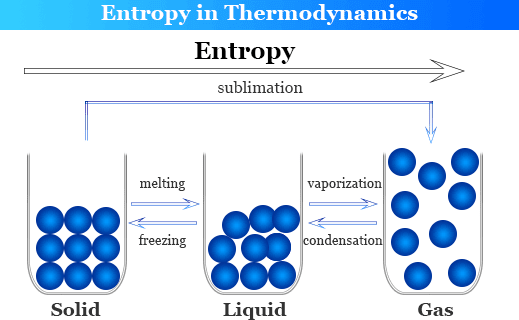
\įrom the balanced equation we can write the equation for ΔS 0 (the change in the standard molar entropy for the reaction): As with other calculations related to balanced equations, the coefficients of each component must be taken into account in the entropy calculation (the n, and m, terms below are there to indicate that the coefficients must be accounted for): The entropy change in a chemical reaction is given by the sum of the entropies of the products minus the sum of the entropies of the reactants. Unlike enthalpies of formation, standard molar entropies of elements are not 0.When comparing standard molar entropies for a substance that is either a solid, liquid or gas at 298 K and 1 atm pressure, the gas will have more entropy than the liquid, and the liquid will have more entropy than the solid.A table of standard molar entropies at 0K would be pretty useless because it would be 0 for every substance (duh!) Standard molar entropy values are listed for a variety of substances in Table T2. the entropy of a pure substance at 298 K and 1 atm pressure). Standard molar entropies are listed for a reference temperature (like 298 K) and 1 atm pressure (i.e.The entropy of a substance has an absolute value of 0 entropy at 0 K. In fact, values for the "standard molar entropy" of a substance have units of J/mol K, the same units as for molar heat capacity. the rise in temperature is the heat capacity, it would seem that in some way, information about the heat capacity (and how it changes with temperature) would allow us to determine the entropy change in a system. Since the quantitative term that relates the amount of heat energy input vs. all the ice has melted or all the liquid has frozen) However, in both of the above situations, the energy change is not accompanied by a change in temperature (the temperature will not change until we no longer have an equilibrium condition i.e. Likewise if a small amount of energy is withdrawn from the system, the equilibrium will shift to the left (more ice).If a small amount of energy is input into the system the equilibrium will shift slightly to the right (i.e.At such a temperature and pressure we have a situation (by definition) where we have some ice and some liquid water.


 0 kommentar(er)
0 kommentar(er)
